News & Events - Details
Types of Laboratory Evaporators
21 February 2022Laboratory evaporation is used to separate either a solid or liquid solute from a prepared sample for analysis by converting the sample's solvent into a vapour. Laboratory evaporators use a variety of techniques to accelerate the evaporation process such as heat, motion, gas, reduced pressure or a combination of two or more of these methods. Evaporators are a staple in many labs, particularly in the pharmaceutical, environmental, food/beverage, academic, and medical fields. Each evaporation method is ideal for certain applications. In this article written by Sarah Johnson, for Organomation, we provide a guide to the most popular laboratory evaporators and their recommended applications.
Nitrogen Blowdown Evaporation
|
|
|
DescriptionNitrogen blowdown evaporation is a widely used method of sample concentration that combines heat with gentle blowdown technology. Nitrogen evaporators maintain a steady stream of nitrogen or compressed air which blows on the surface of the sample solvent. The constant gas flow decreases the vapour pressure just above the sample, preventing any vapour from returning back to the solvent while also creating a vortex motion on the surface of the sample. To further speed up the evaporation process, nitrogen evaporators are often used with a heat source such as a heat block, water bath, or dry bead bath. Recommended applicationNitrogen blowdown evaporators come in a variety of arrays and a number of sample positions making them adaptable for many applications. They are ideal when evaporating a high number of small samples as the evaporation time will be both quick and efficient. The average evaporation time for a 10 mL sample, for instance, is about 20 to 30 minutes. Nitrogen evaporators are not recommended when working with samples larger than 100 mL or solvents with high boiling points due to the prolonged evaporation time Additional components required
Click HERE to learn more about this product |
Rotary Evaporation
|
|
|
DescriptionRotary evaporation is a very popular form of sample concentration that combines rotation, heat, and often times a vacuum. Rotary evaporators (often called “rotovaps”) rotate a flask in a heated water bath, distributing the solvent as a thin film on the interior of the flask and increasing the liquid’s surface area. As the evaporation occurs, solvent vapour travels into either water or dry ice condenser and drips into a collecting flask. The solvent is therefore removed, leaving behind a concentrate in the rotating flask. Rotary evaporators often utilize vacuum pumps as well to further speed up the evaporation process. The use of a vacuum pump reduces the pressure in the liquid-filled flask until it is lower than the vapour pressure of the liquid being used. This causes the solvent to boil at a lower-than-normal temperature, reducing or eliminating the need for a heat source. The merging of both rotation and reduced pressure speeds up the evaporation process while maintaining its effectiveness. Recommended applicationRotary evaporation is ideal for those working with heat-sensitive analytes. Although heat can be used in rotary evaporation, the reduction of atmospheric pressure and the increased surface area of the solvent allow heat to be optional. Rotary evaporation is also best used when concentrating on one large sample flask that is no more than half full. Most models accommodate flasks between 50 mL and 4 L, meaning the recommended sample size for a rotary evaporator is between 25 mL and 2 L. The average evaporation time for the recommended sample sizes is around 5 to 20 minutes. Additional components required
Click HERE to learn more about rotary evaporator. |
Centrifugal Evaporation
|
|
|
DescriptionCentrifugal evaporation utilizes a centrifuge chamber paired with a vacuum pump and solvent condenser. The samples are placed inside the chamber which is hooked up to the vacuum pump. The vacuum pump works by reducing the pressure within the centrifuge chamber, simultaneously reducing the boiling point of the sample’s solvent. There is also a centrifuge rotor that causes the chamber to spin, creating a pressure gradient within the solvent. This pressure gradient allows the samples to boil from the top-down, preventing solvent bumping (bumping refers to the phenomenon where homogenous solvents will overheat and splash out of the tube or vial). As this process continues, the solvent vapour travels to the solvent condenser where it is removed and collected. The tubes within the centrifuge chamber are then left with just the concentrate. Recommended applicationCentrifugal evaporators have a wide range of sample capacities and are ideal for evaporating large batches of small samples at once. Most centrifugal evaporators can accommodate sample sizes between 1 to 50 mL. The average evaporation time for a 10 mL sample can be anywhere from 45 minutes to 3 hours depending on the solvent used. They are also ideal when working with heat-sensitive solvents since the vacuum pump reduces or eliminates the need for applied heat. It is very important to keep samples balanced and to use proper centrifuge tubes to ensure that they don’t break under centrifugal force, especially when working with hazardous solvents. Additional components required
|
Kuderna-Danish Solvent Evaporation
|
|
|
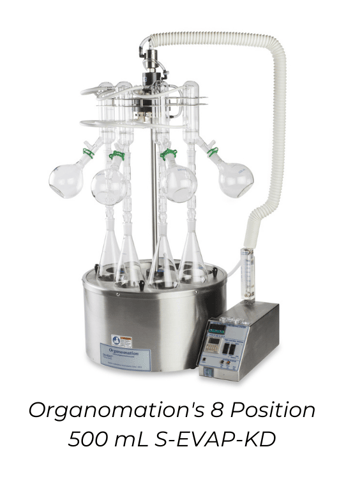
DescriptionSolvent evaporation using Kuderna-Danish flasks is a widely-used method for retaining semi-volatile analytes during concentration and can be paired with solvent collection glassware to reclaim evaporated solvent. The process starts by heating up a flask of solvent to its boiling point. The solvent vapour refluxes in a Snyder column, which is a piece of glass that contains hollow glass balls on the inside. As the solvent vapour travels up the Snyder column, it condenses and evaporates on these glass balls, increasing the retention of semi-volatile analytes. The more volatile solvent vapour continues upward to the condenser, while the less volatile analytes return to the boiling flask. This process continues over and over until all or most of the solvent vapour has passed through the Snyder column and into the environment or fume hood. The concentrated analyte is then left in the bottom boiling flask. Some Kuderna-Danish setups also include an optional Hopkins condenser for solvent collection. Recommended applicationEvaporation using Kuderna-Danish flasks is highly recommended for use with semi-volatile analytes. The use of the Snyder column and repeated condensing allows for high recoveries of these analytes from a more volatile solvent. Kuderna-Danish flasks are typically between 250 mL and 1 L and should only be filled up to 40-60% capacity. Therefore, the recommended sample size for KD evaporation is ~50 mL to 500 mL. The average evaporation time for a 200 mL sample is between 10-15 minutes. One limitation to evaporating with KD flasks is that it is very difficult to go to a precise endpoint (exactly 1 mL, for instance). If a precise endpoint is required, it’s recommended to transfer the concentrator tube to a blowdown evaporator or other small volume evaporation method for final, controlled concentration. Additional components required
|
Freeze Drying
|
|
|
DescriptionFreeze-drying, otherwise known as Lyophilization, is a very popular form of evaporation and is regarded as one of the safest evaporation techniques for delicate samples. This process starts by having a completely frozen sample that is placed under a very low vacuum pump. The vacuum reduces the pressure within the tube or vial until it is well below the solvent’s triple point. The triple point is the pressure and temperature at which the solid, liquid, and gas phases of the same substance can exist at once. Gentle heat is then applied to the sample which causes the frozen solvent to turn directly into a vapor without passing through the liquid phase. This process is called sublimation. As the heat is continuously applied to the sample in order to speed up the evaporation process, the solvent is collected in a condenser leaving behind the concentrate. Recommended applicationSince freeze-drying uses very minimal heat and vacuum pressure, it avoids two main problems of other evaporation methods: bumping and heat damage. Both the heat and pressure can be controlled very precisely, making it one of the safest evaporation methods. Because of these reasons, freeze-drying is ideal for use with sensitive samples, particularly those that are sensitive to heat. Freeze drying is also ideal when working with flasks between 25 mL and 2 L. You shouldn’t fill your flasks more than 1/3 or 1/2 full, making the recommended sample size between ~12 mL and 1 L. The average evaporation time for samples of this size is anywhere from 24 to 48 hours. Samples larger than 1 L can have a much longer evaporation time. Additional components required
|
Reference: Types of Laboratory Evaporators, https://blog.organomation.com/blog/types-of-laboratory-evaporators


 SingaporeSG
SingaporeSG ChinaCN
ChinaCN MalaysiaMY
MalaysiaMY IndonesiaID
IndonesiaID MyanmarMM
MyanmarMM